
Fabrication of a metal free catalyst for chemical reactions through decoration of chitosan with ionic liquid terminated dendritic moiety | Scientific Reports

The crystal structure of dichlorido(1,3-dimesityl-1H-3λ4-imidazol-2-yl)(morpholine-κN)palladium(IV), C25H33Cl2N3OPd

Efficient and Recyclable Solid-Supported Pd(II) Catalyst for Microwave-Assisted Suzuki Cross-Coupling in Aqueous Medium | ACS Omega

US20140294898A1 - Macrocyclic inhibitors of the pd-1/pd-l1 and cd80(b7-1)/pd-l1 protein/protein interactions - Google Patents

N-Heterocyclic Carbene–Palladium(II)–1-Methylimidazole Complex-Catalyzed Suzuki–Miyaura Coupling of Aryl Sulfonates with Arylboronic Acids | The Journal of Organic Chemistry
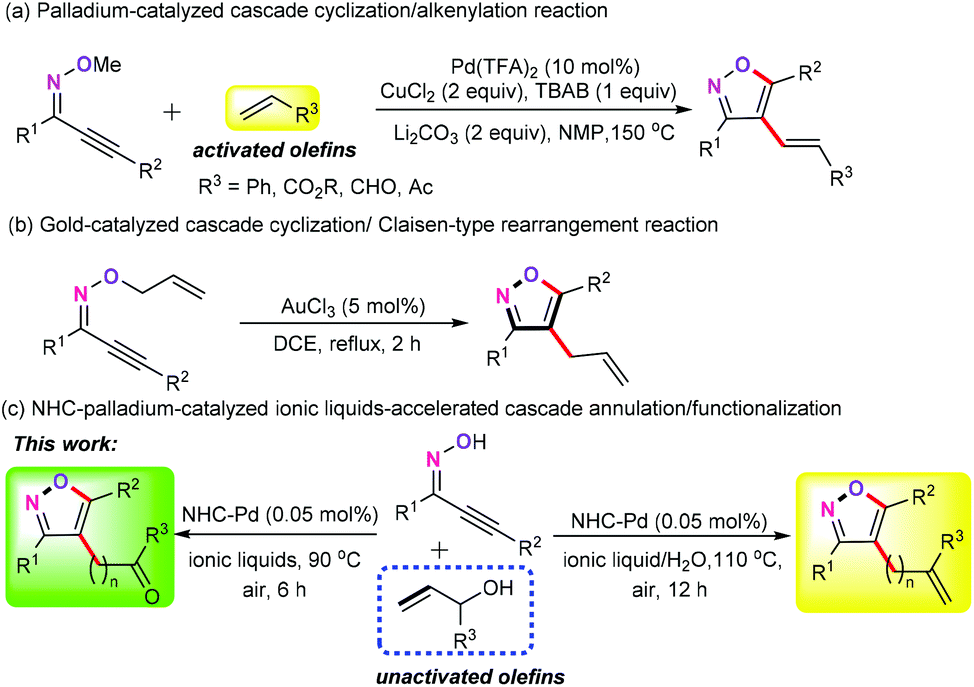
Palladium-catalyzed ionic liquid-accelerated oxidative annulation of acetylenic oximes with unactivated long-chain enols - Green Chemistry (RSC Publishing) DOI:10.1039/D0GC02037K

A suitable modified palladium immobilized on imidazolium supported ionic liquid catalysed transfer hydrogenation of nitroarenes - ScienceDirect

Recent Advances in Pd‐Catalyzed Cross‐Coupling Reaction in Ionic Liquids - Li - 2018 - European Journal of Organic Chemistry - Wiley Online Library

A suitable modified palladium immobilized on imidazolium supported ionic liquid catalysed transfer hydrogenation of nitroarenes - ScienceDirect
![Cross-Coupling Methods for the Large-Scale Preparation of an Imidazole−Thienopyridine: Synthesis of [2-(3-Methyl-3H-imidazol-4-yl)- thieno[3,2-b]pyridin-7-yl]-(2- methyl-1H-indol-5-yl)-amine | Organic Process Research & Development Cross-Coupling Methods for the Large-Scale Preparation of an Imidazole−Thienopyridine: Synthesis of [2-(3-Methyl-3H-imidazol-4-yl)- thieno[3,2-b]pyridin-7-yl]-(2- methyl-1H-indol-5-yl)-amine | Organic Process Research & Development](https://pubs.acs.org/cms/10.1021/op0340457/asset/images/op0340457.social.jpeg_v03)
Cross-Coupling Methods for the Large-Scale Preparation of an Imidazole−Thienopyridine: Synthesis of [2-(3-Methyl-3H-imidazol-4-yl)- thieno[3,2-b]pyridin-7-yl]-(2- methyl-1H-indol-5-yl)-amine | Organic Process Research & Development

Ligand‐Controlled Palladium‐Catalyzed Carbonylation of Alkynols: Highly Selective Synthesis of α‐Methylene‐β‐Lactones - Ge - 2020 - Angewandte Chemie International Edition - Wiley Online Library
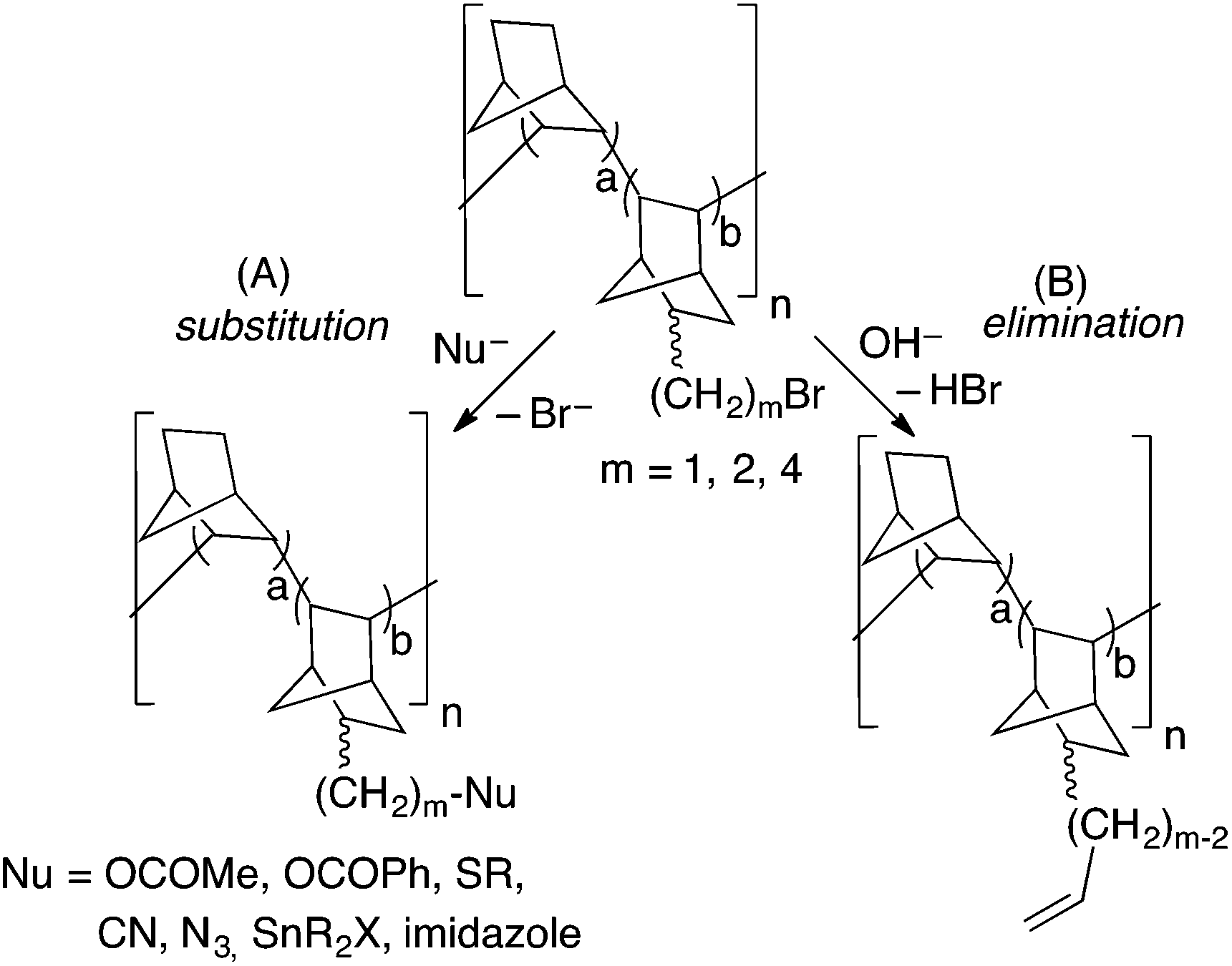
Vinylic addition poly(norbornene- co -alkenylnorbornenes) synthesized with benzylic palladium catalysts: materials for manifold functionalization - Polymer Chemistry (RSC Publishing) DOI:10.1039/D2PY00643J
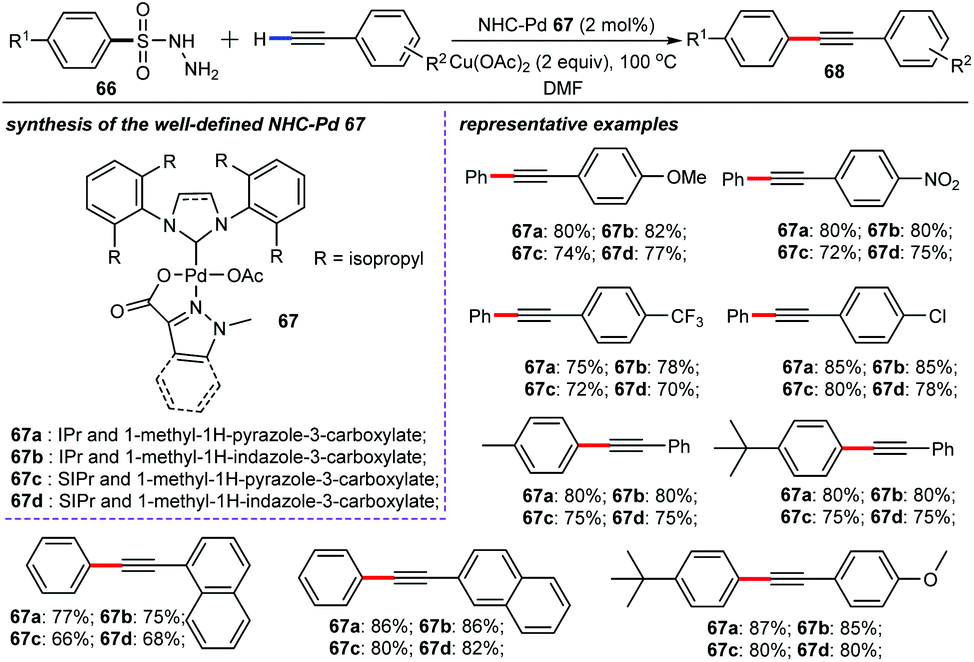
Recent advances in NHC–palladium catalysis for alkyne chemistry: versatile synthesis and applications - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/D1QO00111F

Polymers | Free Full-Text | High Efficiency Gas Permeability Membranes from Ethyl Cellulose Grafted with Ionic Liquids

Shape-Controllable Formation of Poly-imidazolium Salts for Stable Palladium N-Heterocyclic Carbene Polymers | Scientific Reports

1,3-Bis(carboxymethyl)imidazolium Chloride as a Metal-Free and Recyclable Catalyst for the Synthesis of N-Allylanilines by Allylic Substitution of Alcohols | ACS Sustainable Chemistry & Engineering

Recent Advances in Pd‐Catalyzed Cross‐Coupling Reaction in Ionic Liquids - Li - 2018 - European Journal of Organic Chemistry - Wiley Online Library

Imidazolium-urea low transition temperature mixtures for the UHP-promoted oxidation of boron compounds - ScienceDirect

Polymer-Supported N-Heterocyclic Carbene−Palladium Complex for Heterogeneous Suzuki Cross-Coupling Reaction | The Journal of Organic Chemistry

A suitable modified palladium immobilized on imidazolium supported ionic liquid catalysed transfer hydrogenation of nitroarenes - ScienceDirect
Catalysts | Free Full-Text | Immobilized Palladium Nanoparticles on Zirconium Carboxy-Aminophosphonates Nanosheets as an Efficient Recoverable Heterogeneous Catalyst for Suzuki–Miyaura and Heck Coupling

Optically Active (Peptido‐carbene)palladium Complexes: Towards True Solid‐Phase Combinatorial Libraries of Transition Metal Catalysts - Jensen - 2008 - European Journal of Organic Chemistry - Wiley Online Library

![Crystal structure of bis(1-ethyl-3-methylimidazolium) tetrabromidocadmate(II), [C6H11N2]2[CdBr4] Crystal structure of bis(1-ethyl-3-methylimidazolium) tetrabromidocadmate(II), [C6H11N2]2[CdBr4]](https://www.degruyter.com/document/doi/10.1515/ncrs-2015-0117/asset/graphic/j_ncrs-2015-0117_fx_1.jpg)
